
Unlocking the secret to effective cancer treatment? The answer could lie in organ-on-a-chip technology. Researchers of MIMETAS are using organs-on-a-chips to mimic the complex conditions of pancreatic ductal adenocarcinoma. A recent study showed the impact of hypoxia on drugs’ effectiveness, offering potential targets for new drugs.
Researchers often report that a lack of suitable experimental models is hampering the development of new drugs, especially for complex diseases like cancer. A drug might seem very effective in the lab and even show great promise in animal testing. Yet in the full context of the complicated human body, it fails.
One of the plans to overcome this bottleneck is the creation of drug-testing platforms that are truer to nature. With these, chances may be higher that drugs that work in the lab also work in patients.
For about eight years, organ-on-a-chip technologies have shown growing promise to drug developers. At its core is a microfluidic chip. On it, researchers can grow various cell types in 3D cultures and connect the cultures. The chip enables flow conditions that mimic bodily states, such as oxygen pressure, chemical concentration gradients, or fluid shear stress. Both advantages might make organs-on-a-chip the true-to-nature disease model drug developers are looking for.

One company that strives to develop this chip for drug development purposes is MIMETAS. They showcase their technology in a preclinical setting in a recent paper, lead-authored by Marlene Geyer, with bulk RNA sequencing data we could produce.
A drug-testing platform that’s closer to nature
The team focused on pancreatic ductal adenocarcinoma (PDAC), an apt case study for this technology. For this disease, there is no life-saving drug in existence. Gemcitabine is the standard-of-care agent in PDAC, but treatment responses are rarely complete due to resistance and ineffectiveness of the drug. Only around 4% of patients survive two years on the treatment alone. Drug development using canonical model systems seems to be stagnating.
The team at MIMETAS selected two prime features to improve.
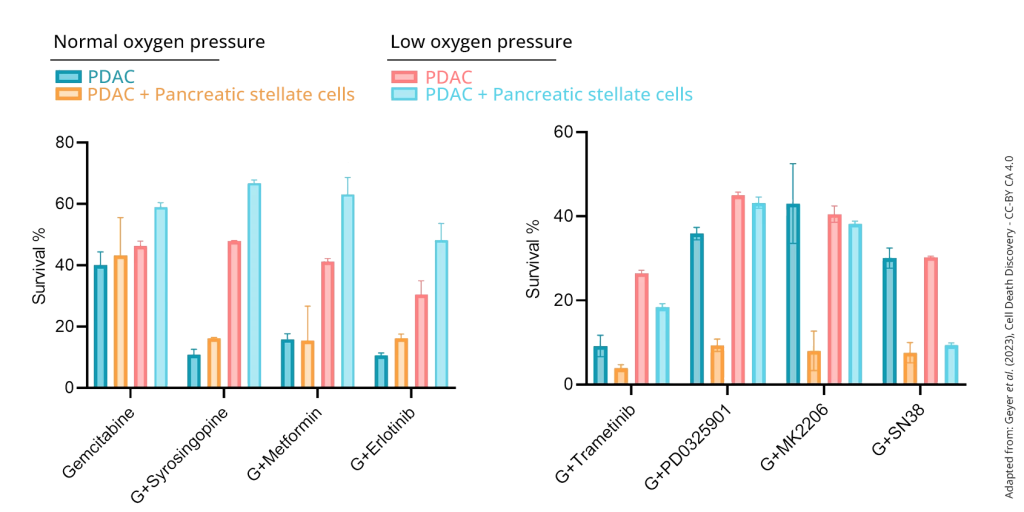
First, PDACs are known to be severely hypoxic. There’s evidence that the low-oxygen conditions contribute to more active tumors that show a higher risk of metastasis, higher chances of drug resistance and immune evasion, and poorer prognosis in patients. It’s likely that the molecular changes happening in hypoxic PDAC make the disease so hard to treat. That’s why Geyer and colleagues focused on a PDAC system that allowed them to modify the oxygen pressure.
Second, 90% of PDAC tumors are high-density stroma. Stroma is the structural, non-cancer cell part of the tumor. It usually consists of structural components such as collagen and many different tumor-interacting cell types, from immune to fat cells. The most studied are the star-shaped pancreatic stellate cells. In response to hypoxia, these cells start producing stroma components that negatively impact the disease outcome. Geyer’s team aimed to create a co-culture with PDAC and pancreatic stellate cells to see how that affected treatment responses. Under the right conditions, the team could successfully create the PDAC drug-testing platform. They used MIMETAS’ advanced OrganoPlate® technology, which enables oxygen pressure modulation and co-culturing of the two cell types as 3D organoids. Four variants were created:

Bulk RNA sequencing
The team leveraged our bulk RNA sequencing services to analyze how hypoxia affected both cell types. We generated gene expression profiles of the samples and provided the data for differential gene expression of cells in low or normal oxygen conditions.
Among other effects, the team saw that hypoxia induces KRAS and TGF-β signaling, apoptosis, and P53 accumulation, as well as a loss of LGR5 and EGFR expression. They also saw that hypoxia triggered epithelial-to-mesenchymal transition genes, which increases the risk for metastasis. These changes happened not only to PDACs but also to the pancreatic stellate cells.
Seeing the transcriptional changes driven by hypoxia gave the team directions on interpreting the subsequent drug test results. It became the team’s aim to understand how modifications observed in hypoxic conditions could contribute to the rise of molecular vulnerabilities to specific drugs.

Drug effects
The team treated their organs-on-a-chip with the standard-of-care gemcitabine plus several additional anticancer drugs. They saw, for example, that the effects of the drug Erlotinib plummeted under hypoxic conditions. This likely is because its target protein, EGFR, was lost in hypoxic cells, as the bulk RNA-seq data showed. Two other drugs’ lack of effect could also be understood by the fact that hypoxia reduced their target gene expression.
Where these drugs were weaker in the hypoxic cancer models, others appeared stronger. For example, the drug Trametinib decreased cell survival in low and normal oxygen levels. It affected monocultures less than co-cultures, while other drugs were deadlier in co-cultures. This suggests that some drug effects operate in the PDAC stroma. Future drugs may exploit both vulnerabilities.
Organs-on-a-chip technology
For anyone used to the gradual pace of drug development, it may come as no surprise that this experiment did not reveal the new PDAC super drug. Regardless, it showed the influence of hypoxia on drugs’ effectiveness. Hypoxia seems to initiate specific molecular programs in PDAC organoids in mono- and co-cultures that impact response to different classes of compounds. It’s better to find that out in the lab than in the later stages of a clinical trial.
This paper directs future studies to include hypoxia mechanisms as possible targets for new drugs. But it also taps into a more considerable development that’s creating more lookalike models for complex diseases.
The organ-on-a-chip technology to accomplish this is quickly evolving. Updated versions of this platform may include more PDAC stroma elements to mimic patient conditions even better. Its technological features may be copied for other tumors in which flow conditions such as oxygen pressure and microenvironment are relevant features. It’s exciting to find out how it will impact drug developers’ success rates. Hence, we’ll keep on following these developments with a keen eye.
You can find the full paper here:


