The University of Sheffield
Mechanism of disease: a new hypothesis for atherosclerosis
Atherosclerosis is seen as the number one cause of death in the developing world. To elucidate the causes of this cardiovascular disease, Sheffield University researchers analyzed the transcriptomes of single endothelial cells. Viewing endothelial heterogeneity in a new way, they could catch sight of a previously unimagined mechanism of disease.

When cells and lipids clot the walls of your blood vessels and your veins and arteries narrow down, it’s bad news. Atherosclerosis, the name for this pattern of disease, starts early in life, but symptoms usually only begin around middle age. In severe cases, it can lead to skin ulcers, kidney failure, heart attack, and stroke. It is the number one cause of death and disability in the world.
Understanding how atherosclerosis occurs is one of cardiovascular science’s great challenges. At the cellular level, atherosclerosis seems to be triggered partly by the cells that line the artery wall, the endothelial cells, getting distraught. How that happens still is unclear.
Studying disturbed blood flow
There seems to be a clue in our blood flow. Researchers have longtime observed that not all regions of the vascular system are affected equally. In straight vessels with an even blood flow, the endothelial cells experience high shear pressure. This friction is a force for good, promoting cellular health and protecting against atherosclerosis.
But on sites where blood vessels branch out and bend, blood flow gets disturbed and complex. In an aortic arch, for instance, the endothelial cells on the outer curvature experience high shear stress, while cells on the inner curve receive low or low oscillatory shear stress. It’s been observed that such disturbed flow conditions promote endothelial cell dysfunction and, consequently, can cause atherosclerosis. But our understanding of why this happens remains murky.

Meet University of Sheffield researchers Dr. Blanca Tardajos Ayllon, postdoc, and Prof. Paul Evans, group leader. The Evans Lab has focused on mechanosensing (how cells sense shear stress) and atherosclerosis for almost twenty years.
For some of their latest research, published in Science Advances, they employed single-cell RNA sequencing to investigate how disturbed blood flow may prompt atherosclerosis. “We discovered the molecular pathway that was involved in atherosclerosis in these regions of disturbed flow,” explains Tardajos Ayllon, who worked together with first author Dr. Celine Souilhol on most of this project. “It seems to operate by regulating endothelial cell heterogeneity.”
Here’s the story of how the Evans Lab got to a new mechanism of disease hypothesis for atherosclerosis.
Identifying the pathway
To uncover the mechanism connecting disturbed blood flow, endothelial cell mechanosensing, and atherosclerosis, the lab focused on the Notch pathway.
“When I was in London ten years ago,” Evans says, “we did some work with pigs. We mapped the flow patterns in the arteries.” The team would take samples from different flow sites, including areas exposed to high shear stress and low oscillatory shear stress. Then, they investigated which genes were differentially expressed in these regions.
“That gave us a lot of hits, which we’ve been taking forward in a number of studies,” he explains. One of these was the pathway of NOTCH4, a mechanoreceptor that is activated by force, and JAG1, its interacting ligand. “It was well known that this pathway was involved in vascular development. But we became the first ones to discover its role in disease.”
Validating JAG1-NOTCH involvement
Evans, Tardajos Ayllon, and colleagues used several complementary experiments to study if JAG1-NOTCH4 became activated in disturbed flow environments. They observed its activation in cultured human endothelial cells, pig aortas, and flow-modified mouse arteries. Both in pigs and mice, JAG1 and NOTCH4 showed higher expression in the endothelial cells of the inner curve—the cells exposed to disturbed flow.

mechanosensitive pathways in vascular endothelium. Adapted from: Souilhol et al. (2020).
The team went on to create a genetic deletion in mice, which specifically cleared the JAG1 protein from endothelial cells. When they put the mice on high-fat diets, atherosclerosis lesions were expected to appear after six weeks. But compared to control mice, the Jag1 knockouts showed significantly fewer lesions.
What’s more, this lesion reduction occurred in the aortic arch, a site of disturbed flow, but not in other areas like the descending aorta or the aortic arch. The JAG1-NOTCH4 pathway appeared to be involved in atherosclerosis in specifically these regions of disturbed flow.
But that was not the full answer. Now was the time to dig even deeper into the mechanism. How does JAG1-NOTCH4 activation lead to endothelial cell dysfunction in these regions?
“We thought we could get to the answer by looking at the impact of Jag1 knockout at the single-cell level,” Tardajos Ayllon says.
Deciding to do single-cell RNA sequencing
Evans explains why single-cell sequencing was the method of choice: “We’re interested in what’s essentially a focal disease.”
“In the aorta, most of the endothelial cells are in the outer curvature, normally exposed to high shear stress, i.e., protected from atherosclerosis. So, if were to take mouse aortas and just flush through for the entire aorta, if we’d, in other words, do bulk RNA sequencing, then the cells we're interested in will be masked. But with single-cell RNA sequencing, you can find and analyze these low-shear cells. That’s made the technology particularly interesting for our research.”
“Single-cell RNA sequencing was a very new technology at the time of the experiment, and I was extremely interested,” Tardajos Ayllon adds. A colleague at the University of Cambridge, Helle Jørgensen, had done single-cell RNA sequencing before. “She told us about the different possibilities and helped us to find out which one best suited our research,” she says. “Then I reached out to Single Cell Discoveries for further advice.”

Advantages of SORT-seq in cardiovascular research
SORT-seq turned out the be the best fit for this project. This plate-based single-cell sequencing method is modular and compatible with FACS sorting. Tardajos Ayllon: “We already knew we were interested in endothelial cells, and we could FACS sort the cells beforehand. This way, the level of contamination with other cells like smooth muscle cells, fibroblasts, and macrophages was really, really small.” With SORT-seq, they could select the cells of choice and so reduce noise.
Another advantage was its compatibility with low cell numbers. “Mouse aortas are really, really tiny,” she explains. “So we wouldn't be able to get many cells from those aortas. 10x Genomics would become difficult because you would need a lot of cells to get a good read and high-quality results. Since we’re talking about 400 endothelial cells from one aorta, the 384-well plate–based SORT-seq was a great match.”
Pilot experiments
“At first, it seemed like a big challenge to start this new approach,” Evans adds. “We were the first in the Sheffield University Medical School to do this. But it seemed feasible once we knew how the system worked. What helps is that Single Cell Discoveries sends over a plate where the lysing buffer is already in place. And for the sequencing part, you got a good track record. So, really, if we could get the FACS sorting optimized, then we had a good chance of getting the data through.”
Tardajos Ayllon performed a few sorting trials on healthy aortas to start with. “It was challenging to optimize the FACS protocol because the Sheffield facility had not done sorting for single-cell experiments before. I had to have a few trials to calibrate the machine and make sure the individual cells went into individual wells. That took some commitment.”
Using microscopy and PCR experiments on random wells, they checked whether sorting was successful. Evans: “These little things build up the confidence to finally say, ‘Right. We're going to get our knock-out mice go for the technical pipeline as we now created it.’”
“I had never done any such experiments before,” Tardajos Ayllon says, so the pilots were a great way to get familiar with the technology. “Still, I remember on the day before doing the first FACS sort, I panicked completely. Will we have enough cells? I remember I emailed one of the people in Single Cell Discoveries: ‘Oh, my, can we have a last-minute meeting?’ And we had that meeting, I think, like at 7:30 or 8:00 in the morning. I mean, that was very helpful. You could reassure me that all was going to be alright.”
Elucidating the mechanism of disease
The team ultimately performed a successful SORT-seq on a total of 991 endothelial cells from Jag1 knockout and control mice. Evans: “That was great. It was very exciting to see that data come through.”
Tardajos Ayllon went to analyze the single-cell data. “One of the things that attracted me to Single Cell Discoveries,” she explains, “is that you send preliminary data analysis results. The fact that you had a collaboration with BioTuring was also extremely useful.” BioTuring’s BBrowserX is user-friendly software to visualize single-cell data, change parameters, and perform manual data analysis. “Although I am no bioinformatician, I could actually go through my data and analyze it, look at it, and test hypotheses without having to depend on a bioinformatician.”
What she found was an unexpectedly heterogeneous mixture of endothelial cells.
“I had seen a couple of papers that previously revealed endothelial heterogeneity,” she says, “but the greatest heterogeneity there, I think, was four clusters maximum.” In contrast, their analysis identified fourteen clusters.
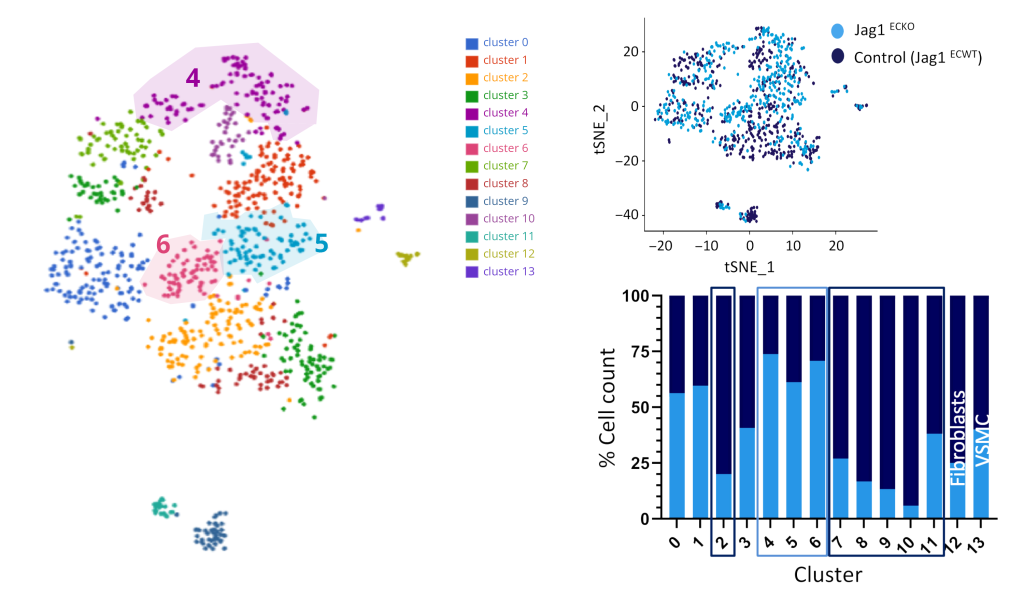
“When I went back to compare with the other papers, I actually saw that the ones they had taken as the main clusters were really variable.” She could find back the same clusters in her data, she says. Tardajo Ayllon: “It matched. But in addition, we could actually find smaller subclusters specific to the endothelial cells we were looking at.”
A novel hypothesis
Then, when they compared cell populations in Jag1 knockout mice with the control, the team observed a key difference. Endothelial cells of Jag1 knockout mice belonged significantly more to one kind of subcluster. The cells showed an expression profile associated with cell proliferation and migration, abilities needed for vascular repair. It indicated that JAG1 has a profound influence on the constitution of endothelial cells and that it suppresses endothelial cells involved in repair.

Importantly, JAG1 knockout experiments in human cultured endothelial cells showed consistent results. Bulk RNA sequencing showed JAG1 inhibited cell division and migration genes in low-shear cultured cells, too. Tardajos Ayllon: “It really improved our story that we could merge the in vivo single-cell data with the in vitro bulk RNA sequencing data.” Later functional experiments showed that Jag1 loss indeed corresponded with increased proliferation of low-shear, but not high-shear, cells.
“Blanca’s work showed that there’re lots of different subsets of endothelial cells in the aorta,” Evans concludes. “And JAG1-NOTCH4 is essential in regulating this heterogeneity.”
Both elements seem to be involved in the pathogenesis of atherosclerosis. Their cumulate hypothesis: low oscillatory shear stress activates JAG1-NOTCH4 signaling, which skews the balance of reparative and pathogenic endothelial cell subsets. Evans: “We think that’s how Notch contributes to atherosclerosis.”

Next steps
“Now that we’ve posed a hypothesis on the mechanism of disease,” Evans continues, “the two next steps would be to find out if there is a way to target that pathway with a therapy, and if that could help.” Although Notch inhibitors exist, there’s no therapeutic that can yet specifically target the JAG1-NOTCH4 pathway in low-shear cells. That’s for future research to uncover.
Regarding single-cell experiments in general, this study has paved the way for more single-cell experiments. “Thanks to Blanca’s efforts,” Evans says, “sample preparation for single-cell experiments is now pretty much routine. So we have got funding to do this for other molecules, addressing their mechanism of disease on a single-cell level.”
“You know, about ten years ago, single-cell sequencing was just the preserve of really specialist labs,” Evans continues. “Now I see it’s becoming a lot more routine. We were able to go from seeing it at conferences to being able to do it ourselves. I think your company was really important for that.”
“It’s maybe not yet completely routine in our group,” Tardajos Ayllon concludes, “but it’s certainly a common technique now. And its possibilities are endless.”
Read the full article here.
How can we help?
Want to supercharge your project with single-cell insights?
Connect with our PhD-level scientists to discuss your biological question, timeline, sample types, and other customizations for your single-cell analysis.
