Leiden Academic Centre for Drug Research
Immune Profiling of atherosclerosis reveals new therapeutic potential
A team of scientists employs 10x Genomics Single Cell Immune Profiling to find and characterize autoreactive T cells in the atherosclerotic plaque. Their results suggest atherosclerosis can be redefined as an autoimmune disease.

Low-cholesterol margarine, whole grain oats, and products like these make up the diet of most at-risk heart disease patients today. The dietary advice is based on the demonstrated scientific fact that atherosclerosis, the number one cause of heart attack and stroke, is a lipid-driven disease.
As such, the established therapeutic approach against atherosclerosis is lowering low-density lipoprotein (LDL) and decreasing blood cholesterol. Cholesterol-lowering foods like the ones mentioned, and drugs such as statins serve that purpose. And they serve it well. In clinical trials, statins reduce the frequency of heart attacks by 25–30% (Endo, 2010). Targeting the lipid aspects of atherosclerosis has likely saved millions of lives.
Still, heart disease and stroke are responsible for more than a fifth of all deaths worldwide (Roth et al., 2020)—enough reason for scientists to look for new therapeutic approaches to strike cardiovascular disease rates down further.
Redefining a disease
For a couple of years, a new line of research has been paving the way for additional atherosclerosis treatment options. Its paradigm-shifting premise is to interpret atherosclerosis as not a lipid disorder but an inflammatory disease.
Groundbreaking work earlier this century has gradually shifted the focus from LDL cholesterol as a driving cause of atherosclerosis to inflammation (Wolf & Ley, 2019). With it followed the plan to therapeutically target inflammation components such as T cells to decrease atherosclerosis burden in patients. Promisingly, clinical trials indeed showed preliminary successes with anti-inflammatory agents (Ridker et al., 2017).
Now, a group of Dutch scientists takes these observations a step further. Their research, recently published in Nature Cardiovascular Research, suggests autoreactive T cells may drive inflammation in atherosclerotic plaques. That would de facto make atherosclerosis an autoimmune-like disease.
New biotherapeutic potential
Dr. Bram Slütter, one of the lead scientists, is principal investigator at the Division of Biotherapeutics at Leiden Academic Centre for Drug Research (LACDR). The team is exploring atherosclerotic plaques from patients to elucidate their immune basis.
“Our ultimate goal for this research is to aid biotherapeutics development,” he explains.
The autoimmunity approach could yield many possible therapeutic strategies. “There are different therapeutic approaches thinkable,” Slütter says. “We may be able to make the pathogenic T cells that are driving this disease less pathogenic. We may eliminate them. Or we may identify protective T-cell subsets that we could induce with vaccination or use to develop CAR T cells, for instance.”
With that in mind, the group employed single-cell RNA sequencing on T cells in atherosclerotic plaques.
“The first objective,” Slütter explains, “was to identify the right target decisively.”

Identifying targetable cells
Atherosclerotic plaques are cholesterol and fat deposits in blood vessels. They are risky pathologies, usually rich in inflammatory cells that can perpetuate and destabilize the plaques until they burst, block arteries, and cause heart attack or stroke.
Dr. Marie Depuydt, one of the paper’s first authors with Dr. Frank Schaftenaar, explains their study’s initiation. “In our lab, we have been working on the role of T cells in atherosclerosis pathogenesis for a long time,” she says.
In previous work with Single Cell Discoveries, they performed single-cell RNA sequencing of the atherosclerotic plaque using the plate-based SORT-seq technology.
“Atherosclerosis-associated inflammation was long considered to be myeloid-driven,” she says. That means the main drivers were mainly macrophages. “But,” Depuydt says, “when we studied the atherosclerotic plaques with SORT-seq, we found that a large part of the immune cells in plaques were actually T cells.”
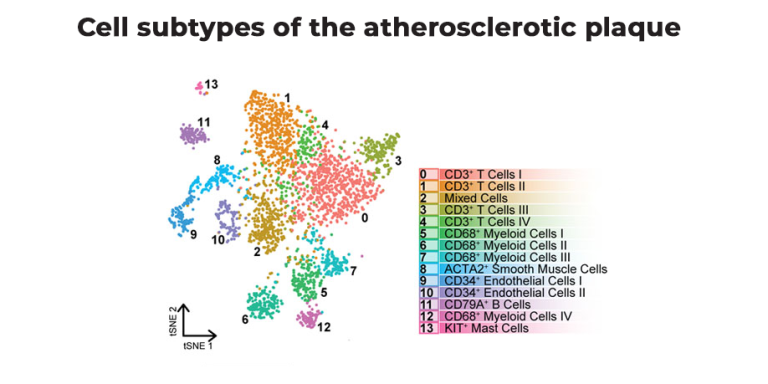
Finding autoreactive T cells
“That made us wonder what activated these T cells,” she says. “Are they directed at the tissue itself? Because that would indeed make it an autoimmune response.”
Slütter continues: “We started to take it apart logically. If atherosclerosis is an autoimmune disease, there needs to be an autoreactive immune response happening on the site of inflammation. If that is the case, there should be autoreactive T cells there.”
The team decided to combine single-cell T receptor sequencing (scTCR-seq) and single-cell RNA sequencing to find and characterize autoreactive T cells, using high-throughput 10x Genomics Single Cell Immune Profiling solution offered by Single Cell Discoveries.
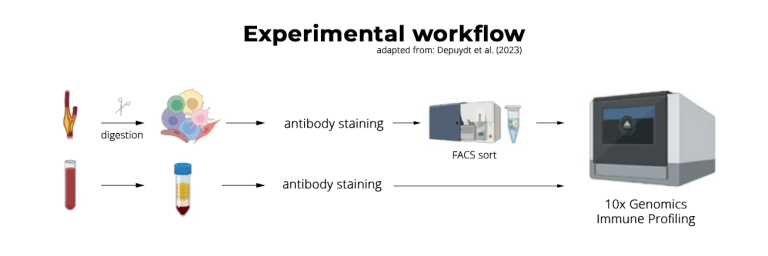
Single Cell Immune Profiling
“For us, as users,” Slütter says, “it’s a great advantage to get the single-cell T cell receptor and RNA information combined for a large number of cells.”
He explains the underlying idea: “Every T cell has a unique T-cell receptor. If you find T cells with the same T-cell receptor, you know they must have originated from a single clone that expanded.”
“With Immune Profiling,” Depuydt continues, “we could see whether the T cells in the plaque clonally expanded. We could also see how much they clonally expanded and, especially, what phenotype the clonally expanded T cells had. That was the main readout to see whether atherosclerosis is indeed an autoimmune-like disease.”
“We collaborated with the lab from Gerard Pasterkamp, who are doing a clinical study on cardiovascular disease patients,” Slütter says. “So they give us material from patients who undergo carotid endarterectomy surgery.” The team analyzed patients’ atherosclerotic plaques and their matched peripheral blood. Slütter: “That way, we can compare the T-cell repertoire in blood and in the inflamed sites of atherosclerosis.”
Working with Single Cell Discoveries
“The peripheral blood cells were quite easy to work with,” Depuydt recounts. Though the atherosclerotic plaque was a different story. “If you look at the atherosclerotic plaque, 95 to 97% of the cells you isolate are dead. That makes it very difficult to isolate enough cells to proceed with single-cell sequencing.”
“We had optimized the digestion protocol before the first single-cell project,” she continues. “Still, it remained trial and error. I couldn’t isolate enough cells the first time. When we increased the number of cells to stain and sort, we eventually did. We had to discuss with Judith Vivié [COO of Single Cell Discoveries] and decided to perform Immune Profiling on the cells without quality control. Because the material was so limited, we couldn’t lose half of it in counting.”
Eventually, it worked.
“To be able to discuss that with you guys was really nice, actually,” she says. “Because there was a lot of trial and error, it helped that there was flexibility on your side, and we had clear communication on what was expected and when to proceed.”
Key findings
Depuydt: “Our first key finding was that we could indeed see these clonally expanded T cells in the human plaque. Then, comparing T cells in plaque and blood, we found an enrichment of especially effector CD4+ T cells in the plaques. They were plaque-specific.”
“When we studied this for the CD8+ T cells,” Depuydt adds, “the difference between plaque and blood was present but less pronounced. So, we decided to move forward with the CD4+ T cells.”
Single-cell RNA sequencing data showed that the clonally expanded T-cell subset expressed genes indicative of recent TCR engagement, such as CD69, FOS, and FOSB.
Together, these findings substantiated that the atherosclerotic plaques harbor one CD4+ T cell subset that regularly undergoes antigen-specific interactions.
“To find these duplicates of clonally expanded T cell receptors in plaque and blood,” she notes, “you really need the large number of cells analyzed by 10x Genomics Immune Profiling.”
Single-cell data analysis
To find out what starts the inflammation, the team employed pseudotime analysis on the single-cell data. Pseudotime analysis is an algorithm that can construct cell trajectories from single-cell data, which shows how cell subsets in a dataset can differentiate into one another.
Using this, the group could pinpoint where the T-cells in the plaque originated. Depuydt: “We found one migratory T cell subset in the peripheral blood samples whose trajectory directed towards the clonally expanded T cells in the plaque.” This subset was likely the precursor population for the antigen-specific CD4+ T cells in the plaque.
The team then employed an analysis tool called CellChat, which can extrapolate receptor-ligand interactions from single-cell data. With it, the team discovered that the plaque-specific T cells interacted mainly with M-TREM2 macrophages, so-called foam cells. “These are very well described to have a prominent role in atherosclerosis as well,” Depuydt concludes, and may be key to how the T cells drive inflammation in the disease.
Comparing the phenotype with an autoimmune disease
With these findings, the team gathered some convincing evidence to characterize human atherosclerosis as an autoimmune-driven T-cell response. But, to further confirm this hypothesis, they integrated their scTCR-seq dataset with a classic autoimmune disease, psoriatic arthritis.
“We projected the psoriatic arthritis T cells on the T cells we found,” Depuydt says. “We could conclude that the clonally expanded T cells in diseased psoriatic arthritis tissue are the same as those we found in the atherosclerotic plaque.”
With that, the team built quite a case to regard atherosclerosis as an autoimmune-like disease.
Future steps
The paradigm shift away from reducing LDL cholesterol levels to targeting T cells in atherosclerosis opens the door for new drug development studies.
Slütter: “Now that we know that autoreactive T cells are present in the atherosclerotic plaque, the next step is to understand what they are reacting to. The step after that is to find out if that mechanism allows us to manipulate the T cells.”
“The next technical step for this is to do spatial transcriptomics,” he says. “For instance, to look at the whole tissue to see where the clonally expanded T cells locate and with what other cells they communicate.”
“With the information we gained with scTCR-seq on the T-cell phenotypes,” Depuydt adds, “we can find out how to eventually target them.”
“We know from previous studies with mice that we can successfully manipulate T cells to reduce atherosclerosis burden,” Slütter says. “Now we need to determine whether this translates to the human situation. This study basically bridges that gap. The big question then becomes: can we manipulate the T cells similarly in a human setting?”
He concludes: “Can we use T cell therapy to reduce atherosclerosis burden in patients?”
If this line of drug development succeeds, at-risk heart disease patients may be adding T-cell vaccines or CAR T medicine to their diet in the future.
Read the full article here.
The image in the header is an artist's impression of the upcoming Leiden University Faculty of Science. Credit: Zes X Zes.
How can we help?
Want to supercharge your project with single-cell insights?
Connect with our PhD-level scientists to discuss your biological question, timeline, sample types, and other customizations for your single-cell analysis.

